How do bats navigate in 3D environments?
The excerpt note is about bat navigation from Yovel & Ulanvosky 2017.
Yovel, Yossi, and Nachum Ulanvosky. “1.18 Bat Navigation.” Learning and Memory: A Comprehensive Reference (2017): 333.
Navigation, the capacity to plan and execute a goal-directed path, is crucial for almost any task an animal must perform, including foraging, roost finding, seasonal migration, and, sometimes even mating. Bats, the only mammal capable of self-propelled flight, show remarkable navigation capabilities on a wide range of spatial scales. Some bat species annually migrate over hundreds or thousands of kilometres, driven by rood availability or heading toward better winter hibernacula. Many bat species commute nightly over dozens to hundreds of kilometres, some location in a remarkably straight line night after night. Many bat species can orient themselves to an exact point in a small-scale space (meters) with a centimetre accuracy, based on proximal and distal landmarks. Despite a dramatic increase in bat navigation research over recent years, our understanding is still sparse in comparison to that of bird navigation; and in particular, relatively little is known about the mechanisms and strategies underlying bat navigation.
In this chapter, the authors summarize the current state of knowledge regarding bat navigation capacity. They divide the navigation strategies into the following types: Beaconing, Route-following, Path integration, Map-based Navigation.
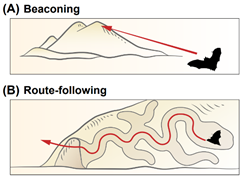
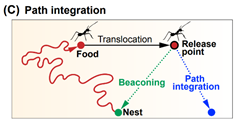
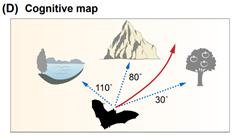

Navigation strategies: schematic illustrations. (A) Beaconing. (B) Route-following. © Path integration. (D) Cognitive map. (E) Map and compass.
Toward a Neurobiology of Natural Navigation in Bats

Navigation-related neurons on small spatial scales. (A) Spatial cell types recorded from the hippocampal formation of Egyptian fruit bats that were crawling on small two-dimensional (2D) surfaces, roughly 1 * 1 m2 in size. From left to right: examples of a place cell, a grid cell, a border cell, and a head-direction cell, all recorded from crawling Egyptian fruit bats. These cell types are very similar to those found in 2D in rodents (Moser et al., 2008). These cell types are thought to be used for the following functions in small-scale navigation: place cells, representing position (map); grid cells, representing positions or distances (odometer); border cells, representing the geometry of the environment; head-direction cells, representing absolute direction (compass). See further details in (Geva-Sagiv et al., 201). (B) examle of a place cell recorded in an Egyptian fruit bat flying in three-dimensional (3D) volumetric space (Yartsev and Ulanovsky, 2013). © Example of a head-direction cell recorded in a bat flying in 3D (Finkelstein et al., 2015). The data in (B, C) were recorded in a large flight room, approximately 6 * 5* 3 m3 in size – much larger than typical small laboratory boxes, but much smaller than real-life navigation distances in bats. To date, it is unknown if these cell types are relevant for large-scale navigation, in either bats or rodents.
The Missing Links II – Neural Mechanisms
How do place cells and grid cells represent large-scale spaces of a few hundred meters or kilometers? And are these cells involved at all in spatial coding of such large spaces? This remains experimentally unknown in any species. In a recent review we provided a detailed account of some of the theoretical possibilities (Geva-Sagiv et al., 2015) – so we will not elaborate on this further here. However, we wish to stress that there are good reasons to believe that the answer must be very different from the spatial codes found, for example, in rodents navigating in small boxes in the laboratory – which makes it vital to record from these neurons on large spatial scales (Geva-Sagiv et al., 2015). Bats provide a particularly good animal model to study these key questions because of their fast flight and ability to cover large distances.
Are border cells relevant to navigation outdoors? Perhaps they may be relevant for navigating inside caves, or along hedgerows – where geometrical borders do existed but are they relevant for bats navigating through open-spaces at high altitudes, where the nearest border may be some cliff a few hundreds of meters away, or further?
How are spatial goals represented in the brain? When navigating from point A to point B, place cells and grid cells are thought to represent the animal’s own position (point “A”) – but what is the neural representation of the goal, and where in the brain it is stored (point “B”)? One proposal is that by activating sequences of place cells, animals may “simulate” their future navigational trajectories (Pfeiffer and Foster, 2013) – but this mechanism could work only for familiar environments where place cells were already established; and additionally it is unclear how it would scale up to larger environments. An alternative possibility is that there might be neurons that represent the vector (direction and distance) to the goal – and could thus implement a neural mechanism for the “home vector” concept mentioned earlier. A recent study has indeed found such a vectorial representation of spatial goals in the hippocampus of bats (Sarel et al., 2017).
How does the brain represent multiple spatial goals? And how does it choose between several possible goals? Namely, what are the neural bases of spatial decision making – in the context of navigation?
What are the neural mechanisms underlying the ability of animals to exhibit reorientation – that is, to find their bearing again after the way was lost? This mysterious ability to lose one’s way but then to reorient again, does not seem to be easily explained by the classical accounts of the various hippocampal-formation spatial cells – and may require more complex mechanisms.
What is the neural mechanism of the long-term maintenance of spatial maps in the bat hippocampus, as well as long-term representation of goals – over months and years? Do fruit bats maintain episodic memory of food items, and if so, what is the neural basis of this capacity – namely, do fruit bats remember which fruit tree (“what”) provides fruit at which location (“where”) and at which season of the year (“when”)? The combination of what–where–when constitutes an episode in episodic like memory, and its neural-level elucidation could link the view that the hippocampus is involved in spatial mapping (O’Keefe and Nadel, 1978) with the view that the hippocampus is a memory circuit, in particular for episodic memories (Eichenbaum and Cohen, 2003).
What are the neural mechanisms of transforming spatial representations from allocentric, absolute spatial coordinates to egocentric, body-referenced coordinates – which in turn could guide locomotion along a navigational trajectory? This question could be studied also in rodents (Wilber et al., 2014); however, bats provide a distinct experimental advantage here because in bats we can track how the animal scans the environment using its sensory gaze. Specifically, we can use an array of microphones to precisely measure the direction of the bat’s sonar beam throughout its flight (Ghose and Moss, 2006; Yovel et al., 2010)dits egocentric “acoustic gaze” – while today it is still rather difficult to measure the eye gaze in freely moving rodents (but see Wallace et al., 2013).
If bats do navigate in groups, as some evidence suggests: What are the neural bases of these capacities? How do bats track the position of one another? How do they transfer information to other individuals? How does their brain weigh and combine their own navigational knowledge with the behavior of the group – for example, does their hippocampal formation perform some sort of Bayesian combination of these two sources of navigational information (Angelaki et al., 2011)? And in particular, how do they decide which bat to follow and which not to?
How do place cells, grid cells, and head-direction cells integrate information across different sensory modalities? Our recent study provided first glimpses into this issue (Geva-Sagiv et al., 2016), but when it comes to navigation outdoors, this becomes a much harder and more complex question because sensory information in real-life conditions varies over both space and time – so it is not entirely clear how it would be used to create a reliable large-scale spatial representation that could underlie real-life natural navigation.
These and many other open questions delineate an uncharted territory in the study of the neural bases of bat navigation and animal navigation more generally. Beyond the border of this uncharted territory, there lie even more complex facets of navigation, and questions that we do not even know how to ask. This might sound as a grim state of affairs. However, the good news are that some dramatic technological advances over the last few years allow now, for the first time, to track the precise position and detailed behavior of animals in natural conditions (Tsoar et al., 2011; Cvikel et al., 2015a, 2015b); as well as to record the activity of single neurons over very large distances and in highly complex environments (Eliav et al., 2016). The next years will prove to be exciting indeed.
For further info, please read the paper Yovel & Ulanvosky 2017.
Yovel, Yossi, and Nachum Ulanvosky. “1.18 Bat Navigation.” Learning and Memory: A Comprehensive Reference (2017): 333.
Alison Abbott. 100 bats and a long, dark tunnel: one neuroscientist’s quest to unlock the secrets of 3D navigation. https://www.nature.com/articles/d41586-018-05648-2
Laboratory of Nachum Ulanovsky. http://www.weizmann.ac.il/neurobiology/labs/ulanovsky/
About
Brain Inspired Navigation Blog
New discovery worth spreading on brain-inspired navigation in neurorobotics and neuroscience
Recent Posts
- How locomotor development shapes hippocampal spatial coding?
- How human, animals, robots encode and recall place?
- How the brain constructs time and space and how these are related to episodic memory?
- How environmental novelty modulate rapid cortical plasticity during navigation?
- How the Hippocampal Cognitive Map Supports Flexible Navigation?
Tags
Categories
- 3D Movement
- 3D Navigation
- 3D Path Integration
- 3D Perception
- 3D SLAM
- 3D Spatial Representation
- AI Navigation
- Bio-Inspired Robotics
- Brain Inspired Localization
- Brain-Inspired Navigation
- Cognitive Map
- Cognitive Navigation
- Episodic Memory
- Excerpt Notes
- Flying Vehicle Navigation
- Goal Representation
- Insect Navigation
- Learning to Navigate
- Memory
- Neural Basis of Navigation
- Path Integration
- Path Planning
- Project
- Research Tips
- Robotic Vision
- Self-Flying Vehicles
- Semantic Memory
- Spatial Cognition
- Spatial Cognitive Computing
- Spatial Coordinate System
- Spatial Learning
- Spatial Memory
- Spatial Resoning
- Time
- Unclassified
- Visual Cortex
- Visual Cue Cells
Links
- Laboratory of Nachum Ulanovsky
- Jeffery Lab
- BatLab
- The NeuroBat Lab
- Taube Lab
- Laurens Group
- Romani Lab
- Moser Group
- O’Keefe Group
- DoellerLab
- MilfordRobotics Group
- The Space and Memory group
- Angelaki Lab
- Spatial Cognition Lab
- McNaughton Lab
- Conradt Group
- The Fiete Lab
- The Cacucci Lab
- The Burak Lab
- Knierim Lab
- Clark Spatial Navigation & Memory Lab
- Computational Memory Lab
- The Dombeck Lab
- Zugaro Lab
- Insect Robotics Group
- The Nagel Lab
- Basu Lab
- Spatial Perception and Memory lab
- The Neuroecology lab
- The Nagel Lab
- Neural Modeling and Interface Lab
- Memory and Navigation Circuits Group
- Neural Circuits and Memory Lab
- The lab of Arseny Finkelstein
- The Epstein Lab
- The Theoretical Neuroscience Lab
- Gu Lab (Spatial Navigation and Memory)
- Fisher Lab (Neural Circuits for Navigation)
- The Alexander Lab (Spatial Cognition and Memory)
- Harvey Lab (Neural Circuits for Navigation)
- Buzsáki Lab
- Brain Computation & Behavior Lab
- ……